Complex Molecular and Biomolecular Systems research team is developing its own RD&I topics and, through collaboration with other groups, provides support for the synthesis / characterization of systems / materials of interest in areas like: health, pharmaceutical and nutraceutical industries, advanced materials, biotechnology, structural biology, biomimetics.
Research topics
- Competitive binding of API’s to plasmatic proteins
- Bioactive compounds (synthetic or natural) with improved solubility and bioavailability by solid form optimization and molecular encapsulation
- Stability studies of bioactive compounds to the action of the excipients and environment
- Structural biology: (i) protein structures by cryo- Electron Microscopy; (ii) structural and dynamical investigations by NMR spectroscopy on isotopically labeled proteins
- Biomimetics: antibacterial effect of natural micropatterned surfaces; identify real-life technological solutions to mimic the natural surfaces
- Cells – nanomaterials interaction: biocompatibility and cito-toxicity studies for various types of nanomaterials
Expertize
- Obtaining new solid forms and inclusion complexes of bioactive compounds (synthetic or natural)
- Surface micro-patterning methods: NIL – Nanoimprint Lithography
- Extraction and purification of protein complexes (soluble and membrane-bound proteins)
- Biocompatibility, cito-toxicity, imunochemistry și and ultrastructural analytical methods for biological samples
- NMR Spectroscopy: on liquids and solids
- X-Ray Diffraction: on single crystals and micro-crystalline powders
- NMR Crystallography
- Electron Microscopy: SEM, TEM, HR-TEM, SAED-TEM
- Elemental analysis: EDX, EBSD
- Atomic Force Microscopy: AFM
- Thermal analysis: DSC, ITC, phototermic techniques, active thermography
- Vibrational Spectroscopy: FT-IR, Raman, VCD
- Molecula modelling: ab initio, DFT – density functional theory molecular mechanics methods on restricted or extended (crystal lattice) systems
- Modelling of complex phenomena: heat propagation in multi-layer systems
Team Leader
Dr. Xenia FILIP – Scientific Researcher II
Expertise: Solid state NMR, NMR crystallography.
Members:
Dr. Lucian BARBU – Scientific Researcher II
Expertise: Electron Microscopy (TEM/SEM/EDX), Cell Biology.
Dr. Diana BOGDAN – Scientific Researcher II
Expertise: Atomic force microscopy, surface characterization.
Marcel BOJAN – Technician
Dr. Gheorghe BORODI – Scientific Researcher I
Expertise: X-ray diffraction, Small angles X-ray scattering, Solid state physics.
Dr. Alexandra CIORÎȚĂ – Scientific Researcher III
Expertise: Biology, Cell cultures, Light and electron microscopy.
Gabriela Lenuța DAMIAN – Technician
Dr. Claudiu FILIP – Scientific Researcher I
Expertise: Solid-state NMR spectroscopy, Spin dynamics – analytical and computational, NMR Cristallography.
Dr. Călin FLOARE – Scientific Researcher III
Expertise: Atomic, Molecular and Chemical Physics, Theoretical Chemistry, Physical Chemistry, Molecular biotechnology.
Dr. Ana Maria Raluca GHERMAN – Scientific Researcher III
Expertise: Molecular and Chemical Physics, Computational Chemistry, Raman Spectroscopy, Multivariate chemometric analysis, Molecular docking.
Dr. Ioana GROSU – Scientific Researcher II
Expertise: synthesis of polymers, organic and coordination compounds, crystallization techniques.
Dr. Irina KACSÓ – Scientific Researcher III
Expertise: Organic chemistry, Thermal analysis – DSC, FTIR spectroscopy.
Dr. eng. Flavia Adina MARTIN – Scientific Researcher II
Expertise: Synthesis of organic compounds, Iterative synthesis of dendritic structures, Solid forms / supramolecular inclusion complexes of bioactive substances, Self-assembled monolayers (SAM) on metal surfaces.
Dr. Elena MATEI – Scientific Researcher II
Expertise: NMR spectroscopy, Biochemistry/ expresion and purification of isotopically labeled proteins, X-ray protein Cristallography, Gold-nanoparticles syntesis/ anti-viral lectins screening.
Dr. Mihaela MIC – Scientific Researcher III
Expertise: Atomic, Molecular and Chemical Physics, Physical Chemistry.
Dr. Maria MICLĂUŞ – Scientific Researcher II
Expertise: X-ray Diffraction, Small-angle X-ray scattering (SAXS), New solids form screening.
Dr. Augustin Cătălin MOŢ – Scientific Researcher II
Expertise: Bioanalytical Chemistry, Chemometry, Bio-spectroscopy.
Dr. Adrian PÎRNĂU – Scientific Researcher II
Expertise: Atomic, Molecular and Chemical Physics, Theoretical Chemistry, Physical Chemistry.
Dr. Sebastian PORAV – Scientific Researcher III
Expertise: .
Cristian SEVCENCU – Scientific Researcher I
Expertise: .
PhD student Andrea SIMION – Research Assistant
Expertise: Solid State NMR spectroscopy.
Dr. Maria SUCIU – Scientific Researcher III
Expertise: Cell Biology.
Eng. Septimiu TRIPON – Technological Development Engineer
Expertise: Transmission and scanning electron microscopy, EDX analysis, preparation and processing all types of electron microscopy samples.
Iulia Teodora VARGA-KOCSIS – Technician
Fundamental studies on polydopamine (PDA) structure and adhesion
Work group: Claudiu Filip, Jürgen Liebscher, Anca Petran, Diana Bogdan, Xenia Filip, Ioana Grosu, Monica Cîrcu, Andrea Simion, Ana-Maria Raluca Gherman
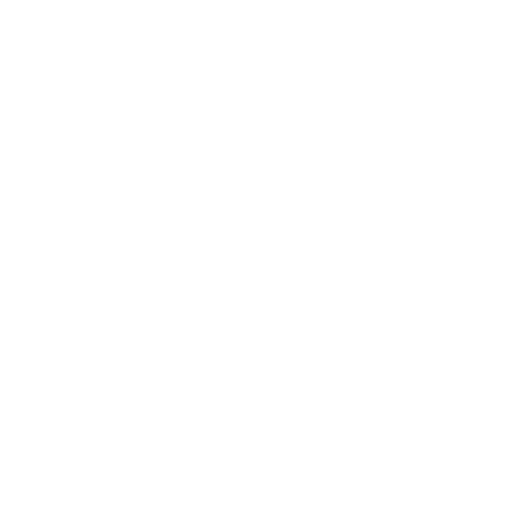
 To date, neither the exact structure nor the mechanisms by which PDA adheres so strongly to virtually any substrate are fully known. Our group has been addressing this issue by performing fundamental research at molecular scale in bulk and ultrathin films of PDA and PDA analogues by solid-state NMR in natural abundance and selectively 13C/15N/2H isotopically labelled samples, and by AFM and SEM/TEM to correlate the findings with the morphology. So far, these studies have been performed within two dedicated research projects1,2 and generated a series of noticeable results, among which we mention: (i) most probable connectivity among major structural units in PDA3 and PDA analogues4, (ii) the dynamics of water molecules inside PDA3, (iii) correlation between the deposition conditions, such as time, pH, oxidizing agent, and the morphology / thickness of the deposited PDA coating layer5, the role of aliphatic sidechain, including the amine group, to the strong PDA adhesion to substrates6.
To date, neither the exact structure nor the mechanisms by which PDA adheres so strongly to virtually any substrate are fully known. Our group has been addressing this issue by performing fundamental research at molecular scale in bulk and ultrathin films of PDA and PDA analogues by solid-state NMR in natural abundance and selectively 13C/15N/2H isotopically labelled samples, and by AFM and SEM/TEM to correlate the findings with the morphology. So far, these studies have been performed within two dedicated research projects1,2 and generated a series of noticeable results, among which we mention: (i) most probable connectivity among major structural units in PDA3 and PDA analogues4, (ii) the dynamics of water molecules inside PDA3, (iii) correlation between the deposition conditions, such as time, pH, oxidizing agent, and the morphology / thickness of the deposited PDA coating layer5, the role of aliphatic sidechain, including the amine group, to the strong PDA adhesion to substrates6.
[1] PN3-P4-ID-PCE-2020-1463
[2] COFUND-M-ERANET-3-InsBIOration
[3] Closer to the polydopamine structure: new insights from a combined 13C/1H/2H solid-state NMR study on deuterated samples, M. Cîrcu, C. Filip, Polym. Chem. 9 (2018) 3379-3387
[4] Oxidative Polymerization of 3,4-Dihydroxybenzylamine – The Lower Homolog of Dopamine, A. Petran, C. Filip, D. Bogdan, C. Zimmerer, S. Beck, T. Radu, J. Liebscher, Langmuir 39 (2023) 5610; / Poly-2-aminomethyl-3-(3,4-dihydroxyphenyl)propionamide: From Structure to Proprieties, A. Petran, A.P. Crisan, C. Lar, A. Popa, T. Radu, A. Ciorata, D. Bogdan, M. Silion, C. Filip, ACS Appl. Polym. Mater. 5 (2023) 3370; / Novel Synthetic Dopamine Analogues: Carbon-13/Nitrogen-15 Isotopic Labeling and Fluorescence Properties, C. Lar, S. Radu, E. Gal, A. Falamas, J.Z. Szucs-Balazs, C. Filip, A. Petran, Anal. Lett. 56 (2023) 170
[5] How thick, uniform and smooth are the polydopamine coating layers obtained under different oxidation conditions? An in-depth AFM study, B. Diana, I.G. Grosu, C. Filip, Appl. Surf, Sci. 597 (2022) 153680
[6] Structural comparison between polydopamine precipitate and thin coating layers, down to nanometer film thicknesses, X. Filip, A. Simion, I.G. Grosu, A.M.R. Gherman, C. Lar, C. Filip, Appl. Surf. Sci. 649 (2023) 159190
Crystal structure determination of bioactive compounds by NMR crystallography
Working group: Claudiu Filip, Gheorghe Borodi, Xenia Filip, Maria Miclăuș, Ioana Grosu

The growing field of NMR crystallography have led to significant improvements in the methodology for structural characterization of organic solids, primarily from microcrystalline powders, by combined solid-state Nuclear Magnetic Resonance (ss-NMR), powder X-Ray diffraction (PXRD) and molecular modelling in full crystal. Our group has a long standing expertise in this field[1] and has brought important contributions to both, its methodolgical development and practical applications. The focus was mainly on solving complex structural problems on bioactive compounds, with more than 15 novel crystal structures being reported over the last years for various solid forms of Quecetin[2-5], Lisinopril[6,7], Ketoconazole[8-9], Tadalafil[10] and Prometazin[11]. Among these, there are two particular cases which deserve special emphasis: (i) Lisinopril dihydrate – a pharmaceutical compound with 24 degrees of freedom to be refined, of which crystal structure was determined[6] from powder at a level of accuracy very close to that of single crystal X-Ray diffraction, and (ii) anhydrous Quercetin – a natural bioactive compound with multiple hydroxyl groups, for which NMR crystallography has proven indispensable to correctly identify the hydrogen bonding network[5] which leads to the observed crystal packing pattern.
[1] Hydrogen-Mediated Noncovalent Interactions in Solids: What Can NMR Crystallography Tell About?, I.G. Grosu, X. Filip, M.O. Miclăuş, C. Filip, Molecules 25 (2020) 3757
[2] Optimized multi-step NMR-crystallography approach for structural characterization of a stable quercetin solvate, X. Filip, M. O. Miclăuș, F. Martin, C. Filip, I. G. Grosu, J. Pharm. Biomed. Anal. 138 (2017) 22-28
[3] Highly sensitive solid forms discrimination on the whole tablet of theactive ingredients in quercetin dietary supplements by NMR crystallography approaches, M. O. Miclăuș, X. Filip, C. Filip, F. A. Martin, I. G. Grosu, J. Pharm. Biomed. Anal. 124 (2016) 274–280
[4] Can the conformation of the flexible hydroxyl groups be constrained by simple NMR crystallography approaches? The case of quercetin solid forms, X. Filip, C. Filip, Solid State NMR 65 (2015) 21-28
[5] NMR crystallography methods to probe complex hydrogen bonding networks: application to structure elucidation of anhydrous quercetin, X. Filip, I. Grosu, M. Miclăuș, C. Filip, CrystEngComm 15 (2013) 4131-4142
[6] Optimizing structure determination from powders of crystalline organic solids with high molecular flexibility: the case of lisinopril dihydrate, M. Miclăuș, I. Grosu, X. Filip, C. Tripon, C. Filip, CrystEngComm 16 (2014) 299-303
[7] Testing the limits of sensitivity in a solid-state structural investigation by combined X-Ray Powder Diffraction, Solid-State NMR and molecular modelling, X. Filip, Gh. Borodi, C. Filip, Phys. Chem. Chem. Phys. 13 (2011) 17978-17986
[8] Ketoconazole-p-aminobenzoic Acid Cocrystal: Revival of an Old Drug by Crystal Engineering, F. Martin, M. Pop, I. Kacso, I.G. Grosu, M. Miclăuş, D. Vodnar, I. Lung, G.A. Filip, E.D. Olteanu, R. Moldovan, A. Nagy, X. Filip, I. Bâldea, Mol. Pharmaceutics, 17 (2020) 919-932
[9] Ketoconazole Salt and Co-crystals with Enhanced Aqueous Solubility, F.A. Martin, M.M. Pop, Gh. Borodi, X. Filip, I. Kacso, Crystal Growth&Design, 13 (2013) 4295-4304
[10] Crystal Structure and Desolvation Behaviour of the Tadalafil Monosolvates with Acetone and Methyl Ethyl Ketone, M. O. Miclăuș, I. E. Kacso, F. A. Martin, L. David, M.M. POP, C Filip, X. Filip, J. Pharm. Sci., 104 (2015) 3782–3788
[11] Distinct Disordered Forms of Promethazine Hydrochloride: A Case of lntergrowth of Polymorphic Domains?, G. Borodi, M.M. Pop, O. Onija, X. Filip, Crystal Growth&Design, 12 (2012) 5846-5851
Characterization of the intermolecular interactions between bioligands and macromolecules
Working grup: Adrian Pîrnău, Călin Gabriel Floare, Mihaela Mic, Mircea Bogdan
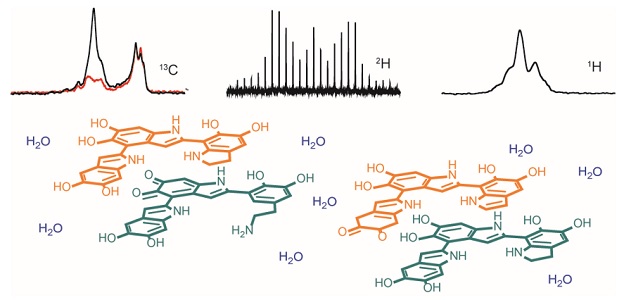
Our current research interests are directed towards understanding the mechanisms of interaction between bioligands and macromolecules (plasma proteins, cyclodextrins) in the liquid state, which allow the characterization of molecular systems in its dynamic evolution, using spectroscopic 1H NMR and calorimetric (ITC) techniques, as well as ab initio calculations and molecular dynamics simulations.
The study of the interaction between drugs and proteins is an important issue from a biochemical and clinical point of view. Transport of drugs in the body, take place via the circulatory system. The plasmatic proteins have ability to bind and transport a diverse range of drugs, metabolites and organic compounds.
Using the techniques mentioned previously, we determined the stoichiometry and the association constant between imatinib (a selective tyrosine kinase inhibitor) and α-1 glycoprotein (AGP), the key parameters specifying the drug affinity to protein[1]. Cyclodextrins are also used as drug transporters to optimize the controlled release of the drugs. Thus, the inclusion complex of β-cyclodextrin (β-CD) with catechol hydrazinyl-thiazole derivative (CHT) with strong antioxidant activity has been characterized in solution.[2].
[1] Study of the binding affinity between imatinib and α-1 glycoprotein using nuclear spin relaxation and isothermal titration calorimetry, Mihaela Mic, Adrian Pîrnău*, Călin G. Floare, Mircea Bogdan, International Journal of Biological Macromolecules 147 (2020) 326–332
[2] Inclusion of a Catechol-Derived Hydrazinyl-Thiazole (CHT) in β-Cyclodextrin Nanocavity and Its Effect on Antioxidant Activity: A Calorimetric, Spectroscopic and Molecular Docking Approach, Mihaela Mic, Adrian Pîrnău*, Călin G. Floare, Mariana Doina Palage, Ovidiu Oniga, Gabriel Marc, Antioxidants 12 (2023) 1367
Methodological developments in solid-state NMR spectroscopy
Working grup: Andrea Simion, Claudiu Filip
In collaboration with Matthias Ernst (ETH Zürich, Elveția), Anne Lesage și Guido Pintacuda (High Field NMR Center, CNRS, Lyon, Franța), Gianluigi Veglia (Universitatea Minnesota, SUA) High-spectral resolution and sensitivity in fast MAS NMR (> 60 kHz) are difficult to obtain using current heteronuclear decoupling sequences. The main drawbacks are the achievement of: (i) robustness for a large chemical shift range under low-power irradiation, (ii) independence with respect to the radio-frequency (RF) power, and (iii) robustness toward radio-frequency field inhomogeneities.
High-spectral resolution and sensitivity in fast MAS NMR (> 60 kHz) are difficult to obtain using current heteronuclear decoupling sequences. The main drawbacks are the achievement of: (i) robustness for a large chemical shift range under low-power irradiation, (ii) independence with respect to the radio-frequency (RF) power, and (iii) robustness toward radio-frequency field inhomogeneities.
Recently, we introduced a new heteronuclear decoupling pulse sequence for fast MAS NMR, that overcomes these issues, dubbed Rotor-Synchronized Phase-Alternated Cycles (ROSPAC) [1,2]. Its advantages were illustrated by representative solid-state NMR experiments and theoretical results obtained by using a generalized theoretical framework based on Floquet theory. Further developments to enhance the decoupling sequences’ efficiency are under consideration, including the design of optimized RF pulses using artificial intelligence (AI).
[1] Heteronuclear decoupling with rotor-synchronized phase-alternated cycles, A. Simion, T. Schubeis, T. Le Marchand, M. Vasilescu, G. Pintacuda, A. Lesage, C. Filip, J. Chem. Phys. 157 (2022) 014202
[2] The effect of 1H offset and flip-angle on heteronuclear decoupling efficiency in ROSPAC pulsed sequence: A Floquet description, A. Simion, M. Ernst, C. Filip, J. Chem. Phys. 158 (2023) 154113
Screening for new solid forms of bioactive compound by high-throughput crystallization
Working group: Irina Kacsó, Ioana Grosu, Flavia Martin, Maria Miclăuș, Gheorghe Borodi, Augustin Moţ
Collaboration with Conf. Dr. Ioana Baldea group, Iuliu Hațieganu University of Medicine and Pharmacy in Cluj-Napoca
Drugs or dietary supplements development is a complex, expensive and long-term process, within which the optimization of the solid form of the active ingredient represents an important step. The selected solid form directly influences essential properties of a bioactive compound: solubility, stability and bioavailability. Thus, new solid forms screening (polymorphs, salts, co-crystals, hydrates/solvates) is an important process in the development of the final product. New solid forms screening, using high-throughput crystallization techniques, offers the possibility of using a varied range of crystallization methods, ensuring the necessary experimental diversity. Thus, the probability of obtaining stable solid forms is enhanced.
The research group’s expertise led to the preparation and characterization of new solid forms for different classes of active pharmaceutical ingredients[1,2], among these some having enhanced stability and solubility vs the active ingredient available on the market. Such an example is Ketoconazole (KTZ): due to a crystal engineering study, performed with the aim to increase its’ solubility, spectacular results were obtained for a series of binary crystalline systems with di-carboxylic acids. The salt of Ketoconazole with oxalic acid and the co-crystal with fumaric acid present a solubility of 50 and 100 respectively times higher than Ketoconazole[3,4].
In order to evaluate the market potential of solid forms with enhanced characteristics (cocrystals of KTZ with fumaric acid or p-aminobenzoic acid, the supramolecular complex of KTZ with PAMAM-G5-NH2 dendrimer[5]), the following are essential: i) the study of compatibility and stability with excipients[6], an important step in drug preformulation; ii) testing the in vivo biocompatibility on an animal model to determine clinical, hematological, biochemical, and histological parameters[7,8]; and iii) determining the pharmacokinetic profile to highlight the improvement of oral bioavailability of the newly obtained solid forms[7]. The in vivo tests were conducted at UMF Cluj.
[1] Structural studies of various olmesartan solvates, I. Grosu, F. Martin, A. Turza, M. Miclaus, I. Kacso, G. Borodi, Acta crystallographica – Section C 78 (2022) 240–249
[2] Exploring the polymorphism of selective androgen receptor modulator YK11, A. Turza, G. Borodi, M. Miclaus, M. Muresan-Pop, Journal of Molecular Structure, 1273 (2023), 134281
[3] Ketoconazole Salt and Co-crystals with Enhanced Aqueous Solubility, Flavia A.Martin, Mihaela M. Pop, Gheorghe Borodi, Xenia Filip, Irina Kacsó, Cryst. Growth Des. 13 (2013) 4295–4304
[4] Cerere Brevet OSIM nr. A/00408 din 15.07.2021, F. Martin, I. Kacso, X. Filip, I. Grosu, M. Pop, “Cocristal Ketoconazol – Acid Adipic 1:1 cu solubilitate crescută pentru administrare orală”
[5] Cerere Brevet OSIM nr. A/00385 din 2.07.2023, F. Martin, I. Baldea, M. Miclaus, I. Grosu, I. Kacso, A. Moț, “Complex supramolecular Ketoconazol-PAMAM-G5-NH2 pentru administrare topică”
[6] Solid-state compatibility studies of Ketoconazole-Fumaric acid co-crystal with tablet excipients, I. Kacso, L. M. Rus, F. Martin, M. Miclaus, X. Filip, M. Dan, J. Therm. Anal. Calorim. 143 (2021) 3499–3506
[7] Ketoconazole-p-aminobenzoic Acid Cocrystal: Revival of an Old Drug by Crystal Engineering, F. Martin, M. Pop, I. Kacso, I. Grosu, M. Miclaus, D. Vodnar, I. Lung, G.A. Filip, E.D. Olteanu, R. Moldovan, A. Nagy, X. Filip, I. Baldea, Mol. Pharmaceutics 17 (2020) 919–932
[8] Ketoconazole-p aminobenzoic cocrystal, an improved antimycotic drug formulation, does not induce skin sensitization on the skin of BALBc mice”, S. Danescu, G. A. Filip, R. Moldovan, D. Olteanu, A. Nagy, X. Filip, F. Martin, I. Kacso, I. Baldea, Inflammopharmacology 29 (2021) 3721–733
Isotopic labelling of γD-crystallin and its study using liquid NMR spectroscopy
Working group: Elena Matei, Călin Gabriel Floare, Adrian Pîrnău, Mihaela Mic

Crystallins, the predominant structural proteins in the eye lens, are the major contributors to its optimal refractive index necessary to focus light correctly into the retina. They must remain stable and soluble at very high concentrations (~400 mg/ml) for the entire life. Unfortunately, with advancing age or due to some congenital factors, α, β, γ -crystallins degrade and form light scattering aggregates resulting in the formation of cataract, the leading cause of blindness worldwide.
In an attempt to study at the molecular level and to identify a non-invasive remedy for cataract, an extensive research using liquid state NMR spectroscopy, and an in silico screening of potential natural inhibitors is performed. The initial work was dedicated to express the recombinat 15N isotopically labelled human γD-crystallins wild-type version (hγD-WT), and the one presenting a mutation from Proline to Threonine at position 23 (hγD-P23T), identified to cause congenital cataract. Additionally the chaperone protein αB-crystallin (αB-crys), known to help maintaining the supramolecular equilibrium, was also expressed and purified. For protein production, syntetic DNA encoding wild-type protein (hγD-WT), hγD-p23T mutant, and αB-crys, were inserted into pET-14b vector, followed by transformation into non-pathogenic BL21(DE3) expression cells.
In parallel, molecular docking simulation were performed were we compared 13 natural inhibitors studied in the literature with lanosterol, an ingredient present in over-the-counter eye products to prevent cataracts. We are currently studying the intermolecular interactions and macromolecular complexes between hγD-P23T and αB-crys, in the absence or presence of natural aggregation inhibitors using mainly a phased-sensitive 2D 1H-15N-HSQC with presaturation for solvent suppression.
We acknowledge the collaboration with Conf. Dr. László-Csaba Bencze group from Chemistry and Chemical Engineering Faculty, UBB Cluj-Napoca, for the expression and purification of the proteins.
A new experimental model for testing the cardiac effects of molecules and the capability of non-conventional materials to record and induce bioelectrical activity
Working group: Cristian Sevcencu, Alin-Alexandru Andrei
This experimental model is based on the identity between the electrophysiology of the chick embryo myocardium and that of the mammalian and human myocardium1 . Based on that identity, we developed a system for simultaneous recording of monophasic action potentials (MAP) and ECG from chick embryo heart which allows the evaluation of cardiotoxic or cardioprotective effects of molecules in their early phases of development2. The same model can also be used in order to evaluate the capability of non-conventional materials (such as electroconductive hydrogels) to record and induce bioelectrical activity. That model was successfully tested in both directions and is currently routinely used in the electrophysiology lab. The advantages of that model in comparison with classical mammalian animal models are: i) the small price of the chick embryos and the possibility to use a large number of embryos and thus to collect a large volume of experimental data in a short period of time; ii) the easy-to-use capabilities of the model; iii) the lack of ethical constrains3 and the consistence of this model with present demands concerning the use of animals for experimentation4.
. Based on that identity, we developed a system for simultaneous recording of monophasic action potentials (MAP) and ECG from chick embryo heart which allows the evaluation of cardiotoxic or cardioprotective effects of molecules in their early phases of development2. The same model can also be used in order to evaluate the capability of non-conventional materials (such as electroconductive hydrogels) to record and induce bioelectrical activity. That model was successfully tested in both directions and is currently routinely used in the electrophysiology lab. The advantages of that model in comparison with classical mammalian animal models are: i) the small price of the chick embryos and the possibility to use a large number of embryos and thus to collect a large volume of experimental data in a short period of time; ii) the easy-to-use capabilities of the model; iii) the lack of ethical constrains3 and the consistence of this model with present demands concerning the use of animals for experimentation4.
[1] The Electrophysiological Organization of the Embryonic Chick Heart, Lieberman M, Paes de CA, Journal of General Physiology 1965; 49(2):351-363.
[2] Cracking the Egg: Potential of the Developing Chicken as a Model System for Nonclinical Safety Studies of Pharmaceuticals, Bjornstad S et al., The Journal of Pharmacology and Experimental Therapeutics 2015; 355(3):386-396.
[3] Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception, Kollmansperger S et al., Animals (Basel) 2023;13(18).
[4] The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease, Graham ML, Prescott MJ., European Journal of Pharmacology 2015; 759:19-29.
Development of new biologically active compounds
Adrian Pîrnău, Călin Gabriel Floare, Mihaela Mic
Collaboration with Prof. Ovidiu Oniga’s group, Iuliu Hațieganu University of Medicine and Pharmacy in Cluj-Napoca
The collaboration with Prof. Ovidiu Oniga’s group (UMF Cluj-Napoca), a group in which new biologically active compounds are developed, mainly aimed at the structural characterization of the newly synthesized compounds as well as their transport in the body. This collaboration has lasted for over 15 years, and the results obtained from this collaboration have been maximize through many publications in ISI listed journals[1][2].

Polyphenols have attained pronounced attention due to their ability to provide numerous health benefits and prevent several chronic diseases. In this study, we designed, synthesized and analyzed a new water-soluble molecule presenting a good antioxidant activity, namely catechol hydrazinylthiazole (CHT). The characterization of the binding mechanism of CHT and HSA, the main plasma protein which have the ability to bind and transport drugs, was carried out by experimental methods (1H NMR and ITC) as well as molecular docking calculations[3].
[1] Synthesis and anti-inflammatory evaluation of some new acyl-hydrazones bearing 2-aryl-thiazole, C. Moldovan, O. Oniga, A. Pârvu, B. Tiperciuc, P. Verite, A. Pîrnău, O. Crisan, M. Bojita, R.Pop, European Journal of Medicinal Chemistry, 46, 2 (2011) 526 – 534 – over 100 independent citations
[2] Discovery of A Novel Series of Quinazoline–Thiazole Hybrids as Potential Antiproliferative and Anti-Angiogenic Agents, A. Șandor, I. Fizeșan, I. Ionuț, G. Marc, C. Moldovan, I. Oniga, A. Pîrnău, L. Vlase, A.E. Petru, I. Macasoi, O. Oniga, Biomolecules, 14, 218 (2024) 1 – 31
[3] Antioxidant Activity Evaluation and Assessment of the Binding Affinity to HSA of a New Catechol Hydrazinyl-Thiazole Derivative, Mihaela Mic, Adrian Pîrnău*, Călin G. Floare, Raluca Borlan, Monica Focsan, Ovidiu Oniga, Mircea Bogdan, Laurian Vlase, Ilioara Oniga, Gabriel Marc, Antioxidants 11 (2022) 1245.
Electrochemical sensors
Diana Bogdan
Collaboration with Prof. Cecilia Cristea’s group, Iuliu Hațieganu University of Medicine and Pharmacy of Cluj-Napoca
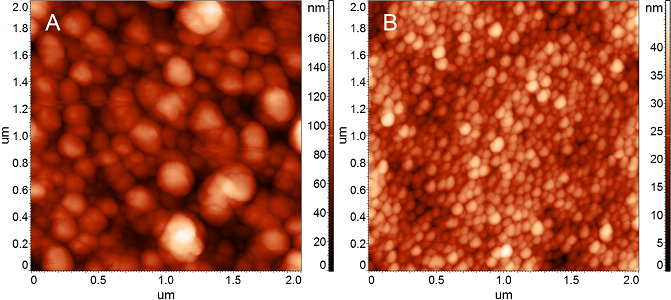
Electrochemical sensors are advancing at a rapid pace due to the high demand, new research in material chemistry and digital communication technologies. In particular, chemically modified electrodes have practical biomedical applications in the development of rapid, sensitive, selective, user-friendly, and noninvasive analysis devices. Electrochemical sensors provide a low-cost and convenient solution for the detection of different analytes and are widely utilized in different industries, in environmental and biomedical applications.
The collaboration with the group of Prof. Cecilia Cristea (UMF Cluj-Napoca), in which such sensors are developed, focused on the characterization of the electrochemical sensors sufaces by atomic force microscopy (AFM), after each modification step: electrochemical sensors for the detection of dopamine[1], electrochemical sensors based on polypyrrole nanoparticles (PPyNPs) decorated with gold nanoparticles (AuNPs) for the selective and sensitive detection of serotonin in real serum samples[2,7], impedimetric aptasensors for the selective detection of Interleukin 6 (IL-6) for colorectal cancer screening[3], electrochemical sensor for the determination of azithromycin in biological samples[4], aptasensors for the sensitive and selective detection of lysozyme (Lyz)[5], aptasensor for on site determination of antibiotics[6], aptasensors for detection of Jurkat cells, which represent tumor cells of T lymphocyte cell leukemia[8], aptasensors for the selective detection of gliadin, the gluten component responsible for triggering celiac disease[9] or aptasensors adapted for the specific detection of tetracycline, for in situ water monitoring[10].
[2] Mihaela Tertiș, Andreea Cernat, Daniela Lacatiș, Anca Florea, Diana Bogdan, Maria Suciu, Robert Săndulescu, Cecilia Cristea: Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture ELECTROCHEM COMMUN 75, 43-47 (2017)
[3] Mihaela Tertiș, Petrică Ionuț Leva, Diana Bogdan, Maria Suciu, Florin Graur, Cecilia Cristea: Impedimetric aptasensor for the label-free and selective detection of Interleukin-6 for colorectal cancer screening BIOSENS BIOELECTRON 137, 123-132 (2019)
[4] Ioan-Adrian Stoian, Bogdan-Cezar Iacob, Cosmina-Larisa Dudaș, Lucian Barbu-Tudoran, Diana Bogdan, Iuliu Ovidiu Marian, Ede Bodoki, Radu Oprean: Biomimetic electrochemical sensor for the highly selective detection of azithromycin in biological samples BIOSENS BIOELECTRON 155, 112098 (2020)
[5] Gheorghe Melinte, Oana Hosu, Geanina Ștefan, Diana Bogdan, Cecilia Cristea, Giovanna Marrazza: Poly-l-Lysine@gold nanostructured hybrid platform for lysozyme aptamer sandwich-based detection ELECTROCHIM ACTA 403, 139718 (2022)
[6] Adrian Blidar, Oana Hosu, Bogdan Feier, Geanina Ștefan, Diana Bogdan, Cecilia Cristea: Gold-based nanostructured platforms for oxytetracycline detection from milk by a “signal-on” aptasensing approach FOOD CHEM 371(3), 131127 (2022)
[7] Mihaela Tertiș, Petra Lia Sîrbu, Maria Suciu, Diana Bogdan, Ovidiu Pană, Cecilia Cristea, Ioan Simon: An innovative sensor based on chitosan and graphene oxide for selective and highly-sensitive detection of serotonin CHEMELECTROCHEM 9(6), e202101328 (2022)
[8] Iulia Rus, Mihaela Tertiș, Anca Pop, Ionel Fizeșan, Diana Bogdan, Elena Matei, Daniela Oprea, Victor Diculescu, Robert Săndulescu, Cecilia Cristea: The use of a new selective AB3 aptamer for the hematologic tumor cells’ detection SENSOR ACTUAT B-CHEM 394, 134389 (2023)
[9] Mihaela Tertiș, Manuela Zăgrean, Alexandra Pusta, Maria Suciu, Diana Bogdan, Cecilia Cristea: Innovative nanostructured aptasensor for electrochemical detection of gluten in food samples MICROCHEM J 193, 109069 (2023)
[10] Oana Hosu, George Melinte, Geanina Ștefan, Magdolna Casian, Cecilia Cristea: Towards selective tetracycline recognition in wastewater based on gold nanovoids@aptamer sensing ELECTROCHIM ACTA 460, 142556 (2023)
Solid-state NMR investigations of polymers
Claudiu Filip, Xenia Filip
Collaboration with: Prof. Jürgen Liebscher, Dr. Alexandrina Nan and Dr. Anca Petran from the research team Multifunctional materials and biologically active compounds
Collaboration with Radosław Mrówczyński, Center for Advanced Technologies, Uniwersytetu Poznańskiego, Poland

The research undertaken by the Multifunctional materials and biologically active compounds team in the field of polymeric materials and polymer composites address, among others, the development of new polymers / co-polymers, preferably biocompatible / biodegradable, with properties tailored for applications in the pharmaceutical industry, depolution and medicine. To comply with the demands specific to these type of practical applications, a detailed structural characterization, as well as the physico-chemical properties knowledge, of these new systems is absolutly neccessary.
In this context, our research team offers its expertise in ss-NMR structural characterization of polymeric materials, bringing important contributions along the time to a series of remarkable results of this research group on polymers, among which we mention: determination of important structural features of polydopamine[1], ss-NMR characterization of some polydopamine analogues[2-4], and of some new biocompatible / biodegradable polymers based on tartaric acid[5], bezofuran-co- arylacetic acid[6] and 4-fluoromandelic acid[7] or copolimers[8].
[1] Structure of polydopamine: a never-ending story?, J. Liebscher, R. Mrowczynski, S.A. Scheidt, C. Filip, N.D. Hădade, R. Turcu, A. Bende, S. Beck, Langmuir, 29 (2013) 10539-10548
[2] Replacing amine by azide: dopamine azide polymerization triggered by sodium periodate, M. Szukowska, Ł. Popenda, E. Coy, C. Filip, J. Grajewski, M. Kempiński, Y. Kim and R. Mrówczyński, Polymer Chemistry 13 (2022) 3325-3334
[3] Poly[3,4-dihydroxybenzhydrazide]: A Polydopamine Analogue?, A. Petran, N. D. Hădade, C. Filip, X. Filip, A. Bende, A. Popa, J. Liebscher, Macromolec. Chem. Phys. 219 (2018) 1700564
[4] Melanin-like polydopa amides – synthesis and application in functionalization of magnetic nanoparticles, A. Petran, R. Mrowczynski, C. Filip, R. Turcu, J. Liebscher, Polym. Chem. 6 (2015) 2139-2149
[5] Clean production of new functional coatings of magnetic nanoparticles from sustainable resources, A.Nan, X. Filip, M. Dan, O. Marincaş, J. Cleaner Prod. 210 (2019) 687-696
[6] Poly(benzofuran-co-arylacetic acid) – a new type of highly functionalized polymers, A. Nan, A. Bunge, M. Cîrcu, A. Petran, N.D. Hădade, X. Filip, Polym. Chem. 8 (2017) 3504-3514
[7] Efficient chemical synthesis of new thermoplastic fluorinated aromatic polyester, A. Nan, R. Teodora, X. Filip, I. Kacso, N.D. Hadade, F. Nekvapil, M. Miclăuş, Polymer 283 (2023) 126261
[8] Development of a New Eco-Friendly Copolymer Based on Chitosan for Enhanced Removal of Pb and Cd from Water, I.V. Ganea, A. Nan, C. Roba, I. Neamtiu, E. Gurzau, R. Turcu, X. Filip, C. Baciu, Polymers 14 (2022) 3735
Design, synthesis and structural characterization of halogen bonded supramolecular complexes
Ioana Grosu, Maria Miclăuș
Collaboration with Organic Chemistry Group, Supramolecular Organic and Organometallic Chemistry Centre – SOOMC, Faculty of Chemistry and Chemical Engineering, Babeș-Bolyai University, Cluj-Napoca, Romania
 Halogen bonding is a versatile tool for accessing a variety of supramolecular architectures, such as host−guest complexes, macrocycles, cryptands, or 1D, 2D, 3D polymeric structures. Due to their significant contribution, with directionality and strength similar to hydrogen bonds, these interactions play a major role in stabilizing supramolecular structures, making halogen bonds significant driving forces for supramolecular self-assembly, gaining considerable interest in the last two decades.
Halogen bonding is a versatile tool for accessing a variety of supramolecular architectures, such as host−guest complexes, macrocycles, cryptands, or 1D, 2D, 3D polymeric structures. Due to their significant contribution, with directionality and strength similar to hydrogen bonds, these interactions play a major role in stabilizing supramolecular structures, making halogen bonds significant driving forces for supramolecular self-assembly, gaining considerable interest in the last two decades.
In collaboration with the Organic Chemistry Group, Faculty of Chemistry and Chemical Engineering, UBB, various supramolecular polymeric systems, with diverse architectures, formed through halogen interactions N—I, X—X, or C(aliphatic)–H—X (X: halogen) were synthesized and structurally characterized.
[1] Halogen-bonded supramolecular architectures involving 2,7-dipyridylfluorene and 1,3,5-trifluoro-2,4,6-triiodobenzene tectons – a spectacular evolution from catemers to 2d halogen bond organic frameworks (XBOF), L. Cata, I.G. Grosu, M. Miclăuș, N.D. Hadade, I. Grosu, A. Terec, Studia Universitas Babeș-Bolyai Chemia, 67 (2022) 193
[2] Halogen-Bonded Organic Frameworks of Perfluoroiodo- and Perfluorodiiodobenzene with 2,2 ‘,7,7 ‘-Tetrapyridyl-9,9 ‘-spirobifluorene, L. Pop, I.G. Grosu, M. Miclăuș, N.D. Hadade, A. Pop, A. Bende, A. Terec, M. Barboiu, I. Grosu, Crystal Growth & Design, 21 (2021) 1045
[3] Halogen Bonds (N—I) at Work: Supramolecular Catemeric Architectures of 2,7-Dipyridylfluorene with ortho-, meta-, or para-Diiodotetrafluorobenzene Isomers, I.G. Grosu, L. Pop, M. Miclăuș, N.D. Hadade, A. Terec, A. Bende, C. Socaci, M. Barboiu, I. Grosu, Crystal Growth & Design, 20 (2020) 3429
[4] The nature of intermolecular interactions in pyridinium-anion-beta-hexachlorocyclohexane molecular crystals, I.G. Grosu, M.I. Rednic, M. Miclăuș, I. Grosu, A. Attila, Physical Chemistry Chemical Physics, 19 (2017) 20691
[5] Supramolecular anion recognition by beta-HCH, M.I. Rednic, R.A. Varga, A. Bende, I.G. Grosu, M. Miclăuș, N.D. Hadade, A. Terec, E. Bogdan, I. Grosu, Chemical Communications, 52 (2016) 12322
Supramolecular systems for improving the physico-chemical properties of natural biologically active compounds
Irina Kacsó, Flavia Martin, Maria Miclăuș, Xenia Filip, Călin Floare, Ioana Grosu, Alexandra Ciorîță, Sebastian Porav
Partnership with Parapharm SRL
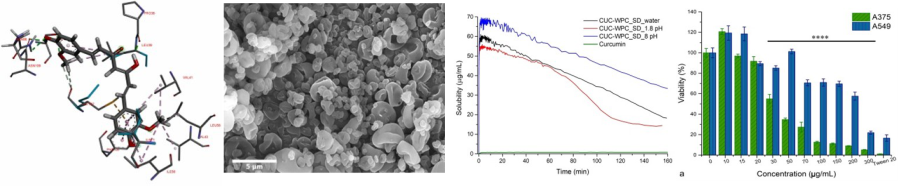
Exploitation of the therapeutic properties of natural compounds is greatly hampered by unfavorable physico-chemical properties of them: low water solubility, stability and bioavailability. Improving these physico-chemical properties by encapsulating of the compounds in various carrier matrices have major importance for the pharmaceutical, nutraceutical and medical device industries.
The our group’s expertise in supramolecular systems development for the natural or synthetic biologically active compounds, using various types of oligo- or polymeric carrier matrices, constituted the basis of collaboration with Parapharm SRL – an important player on the food supplements market.
The result of this collaboration led to the development of Curcumin-Whey Protein supramolecular systems and their physico-chemical and biological characterization.
[2] Complexation of curcumin using whey proteins to enhance aqueous solubility, stability and antioxidant property, L ZS Racz, CS-P Racz, O Horovitz, G Tomoaia, A Mocanu, I Kacso, M Sarkozi, M Dan, S Porav, G Borodi, M Tomoaia-Cotisel, STUDIA UBB CHEMIA, LXVII, 3 (2022) 75-99
[3] Curcumin-whey protein solid dispersion system with improved solubility and cancer cell inhibitory effect, L ZS Racz, M Tomoaia-Cotisel, Cs-P Racz, P Bulieris, I Grosu, S Porav, A Ciorita, X Filip, F Martin, G Serban, I Kacso, STUDIA UBB CHEMIA, LXVI, 3 (2021) 209-224