The research group Isotopic Separation and Labeled Compounds develops theore tical and experimental research related to the physico-chemical processes for the separation of light stable isotopes and the production of isotopically labeled organic and inorganic compounds.
tical and experimental research related to the physico-chemical processes for the separation of light stable isotopes and the production of isotopically labeled organic and inorganic compounds.
Research is aimed at:
- Modeling the behavior of separation columns of carbon, oxygen and argon isotopes using cryogenic distillation
- Obtaining experimental data regarding the functioning of the isotope separation columns in various operating regimes
- Design, construction and experimentation of separation columns and automation of isotope separation processes
- Obtaining light stable isotope labeled compounds with applications in various research fields such as physics, biology, medicine, chemistry, pharmacology.
Team Leader
Dr. József Zsolt SZŰCS-BALÁZS – Technology Development Engineer II
Expertise: Separation processes, Isotope separation, Transport phenomena, Process system engineering, Custom Isotope Labeling, Catalysis and reaction engineering, Cryogenics.
Members:
Dr. eng. Ancuţa BALLA – Recognised Researcher (R2)
Expertise: Inorganic Chemistry, Physical chemistry, Isotopic Separations, Catalysis.
Răzvan BOT – Senior Technician (TS)
Eng. Ştefan BUGEAC – Technology Development Engineer III
Expertise: Industrial engineering, Manufacturing technology, Multi-phase systems, Cryogenics, Experimental mechanics, Vacuum technologies, Special construction know-how, Processing and evaluation.
István CSETE – Senior Technician (TS)
Eng. Mihai GLIGAN – Technology Development Engineer I
Expertise: Separation processes, Isotope separation, Cryogenics, Mechanical design of separation instalations, Gas separation and purification, Vacuum technologies, Heat exchange.
Dr. Claudia LAR – First Stage Researcher (R1)
Expertise: Organic Chemistry, Supramolecular Chemistry, Inorganic Chemistry.
Dr. eng. Cristina MARCU – Recognised Researcher (R2)
Expertise: Isotopic separation by chemical exchange, Physical Chemistry, Anionic exchange.
Cristinel OPREA – Senior Technician (TS)
Orlando PĂTRAŞ – Senior Technician (TS)
Ligia POP – Senior Technician (TS)
PhD student eng. Stelian RADU – Technology Development Engineer
Expertise: Isotope separation processes, Cryogenics.
Dr. Codruţa VARODI – Established Researcher (R3)
Expertise: Applied Electrochemistry; Modified electrodes, Isotopic separations, Gas Chromatographic analyzes, Isotopic analyzes, Analytical chemistry, Physical Chemistry.
 Title: The Production of the Isotope 15N by Isotopic Exchange in Nitrox System at Pressure
Title: The Production of the Isotope 15N by Isotopic Exchange in Nitrox System at Pressure
Journal: Rev. Chim. (Bucharest) 70, 5, p.1530-1533 (2019)
Authors: Damian Axente, Ștefan Bugeac, Ștefan Gergely, Mihai Gligan, Cristina Marcu, Zsolt Szücs, Codruţa Varodi
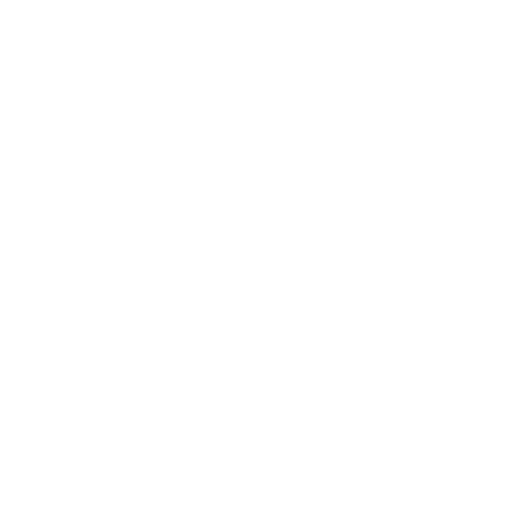
The operation of 15N production plant at pressure of 0.7 +/- 0.05 bar in stable conditions at total reflux and in production is demonstrated. The 10M HNO3 flows used in the primary column were 6.6 L/h and 6.0 L/h, 47, respectively 33% higher than that of atmospheric pressure (4.5 L/h) and corresponding flow rates of the same column were 3.12 mL/cm2 ∙ min, respectively 2.83 mL/cm2 ∙ min for operation at pressure, compared to 2.12 mL/cm2 ∙ min at atmospheric pressure. The 10M HNO3 flows used in the final column were 324 mL/ h and 264 mL/h, 62, respectively 32 % higher than that of the atmospheric pressure (200 mL/h) and flow rates of that column were: 3.05 mL/cm2 ∙ min, respectively 2.49 mL/cm2 ∙ min, compared to 1.86 mL/cm2∙ min for atmospheric pressure operation (Fig. 1). The HETP values are lower for both separation columns operated at pressure and higher 10M HNO3 flow rates, that being an argument in the favour of operation the 15N production plant by isotopic exchange in Nitrox system at pressure. The nitrogen enriched in 15N losses in the waste sulphuric acid, evacuated at the bottom of product refluxer stage II, were : 5.18 -10.58 ppm of feeding with 10M HNO3 of the plant. Any losses of nitrogen enriched in 15N represent an additional uncontrolled production, which diminishes the production of 15N plant. In the case of operation at pressure the flows of H 15NO3 extracted as plant product are higher than those corresponding to atmospheric pressure because the nitric acid solution is chemically equilibrated with nitrogen oxides at the operation pressure and temperature. At a pressure of 1 atm. in the 15N production plant the nitrogen content of nitric acid solution would be 10.919M instead of 10M.
 Title: Method and installation for sulfur dioxide and oxygen recycling on the 15N production plant by isotopic exchange in the system (NO, NO2)(g) – HNO3(s)
Title: Method and installation for sulfur dioxide and oxygen recycling on the 15N production plant by isotopic exchange in the system (NO, NO2)(g) – HNO3(s)
Authors: Axente Damian, Balla Ancuţa Carmen, Marcu Mariana Cristina, Gergely Ștefan
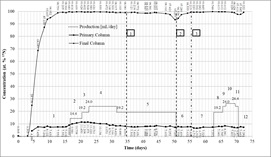
The invention refers to a method and installation for recycling of sulfur dioxide and oxygen in the plant for 15N production by isotopic exchange in the nitrogen oxides in gaseous phase and nitric acid solution (Nitrox). The method, according to the invention, consists in: the concentration of the sulfuric acid, waste of the separation plant, to 96 – 98% by distillation, decomposition at 320oC into sulfur trioxide and water vapors, which pass by a catalytic bed, where takes place the conversion of sulfur trioxide to sulfur dioxide and oxygen. The gaseous mixture is compressed at 15 atm for sulfur dioxide liquefaction, which is collected and then is recycled in the nitrogen oxides refluxers of the 15N separation plant and the gaseous oxygen, separated from the liquid sulfur dioxide, is recycled in the nitric acid refluxer of the 15N separation plant.
The installation according invention (Fig. 2) consists of: reactor (2) with a catalyst bed (3), an electric furnace (4) for heating the catalyst bed, a tank (1) for sulfuric acid, a metering pump (5), a heat exchanger (6) for water vapors condensation, which is collected in the vessel (7) together the unconverted sulfuric acid, a drying tower (8), a compressor (9), another heat exchanger (10), a pressure cylinder (11) for liquid sulfur dioxide collecting and gaseous oxygen separation.
- Dark Side Collaboration – Global Liquid Argon Dark Matter Search Program
- GAPS – Dipartimento di Chimica, Materiali e Ingegneria Chimica “Giulio Natta” at Politecnico di Milano – Group on Advanced Separation Processes & GAS Processing
- Technical University of Cluj Napoca