The Porous materials and carbon nanostructures research group works in the area of preparation, characterization and applications of porous and nanostructured materials and composites such as: ordered porous oxides, metal organic frameworks (MOF), graphenes, N and/or S doped graphenes and their composites with metal nanoparticles.
The main research areas developed in our group are:
- Preparation of nanostructured materials and micro- mesoporous materials such as: MOFs, oxide supported MOFs, ordered porous mixed oxides, reduced graphene oxide (rGO), graphene doped with heteroatom (nitrogen; sulfur), organic functionalized graphenes, MeNP-MOF composites, MeNP-rGO composites
- Synthesis of graphenes/ graphene decorated with metallic nanoparticles by electrochemical exfoliation of graphite rods (prototype)
- Nanostructured and porous materials characterization (structural and functional):
- (i) total surface area and metal surface area,
- (ii) surface reactivity toward several processes by thermoprogrammed analysis,
- (iii) thermal stability by thermogravimetric analysis,
- (iv) gas adsorption capacity (adsorption thermodynamic and kinetic).
- Development of catalytic processes (thermal catalysis, electrocatalysis, photocatalysis):
- (i) CO2 transformation to value added products,
- (ii) superior biogas valorization,
- (iii) hydrogen production and storage,
- (iv) development of electrode materials for fuel cells,
- (v) organic pollutants adsorption and degradation.
- Fabrication of graphene-modified electrodes employed for the electrochemical detection of tumor biomarkers, food additives and organic pollutants.
Team Leader
Dr. Diana LAZĂR – Leading Researcher (R4)
Expertise: Heterogeneous Catalysis, Green Energy, Environmental Catalysis.
Members:
Dr. Gabriela BLĂNIȚĂ – Established Researcher (R3)
Expertise: Organic chemistry, Physical Chemistry, Materials chemistry, Hydrogen technologies.
Dr. Maria COROȘ – Established Researcher (R3)
Expertise: Advanced materials chemistry, Supramolecular chemistry, Natural compounds chemistry.
Eng. Dragoș Viorel COSMA – Scientific Research Assistant (ACS)
Expertise: Nanostructured materials, Photocatalityc aplications.
Dr. Monica DAN– Recognised Researcher (R2)
Expertise: Nanomaterials, Chemistry, Heterogeneous Catalysis.
Dr. Oana GRAD – Recognised Researcher (R2)
Expertise: Chemistry, Materials Science, Organic Chemistry, Heterocyclic Chemistry.
Dr. Camelia GROȘAN – Established Researcher (R3)
Expertise: Organic chemistry, Materials science and nanomaterials, Applied Electrochemistry, Vibrational Spectroscopy.
PhD student Eng. Angela-Maria KASZA – Technological Development Engineer
Expertise: Preparation and characterization of porous materials, Heterogeneous catalysis; metal-organic structures (MOFs).
Dr. Anzarul Haque KHAN – Project Director
Expertise: .
Dr. Maria MIHEȚ – Established Researcher (R3)
Expertise: Heterogeneous catalytic processes involving CO2 and/or H2, Hydrogen – production, chemical storage and utilization, Metal/support catalysts, Metal-organic frameworks in catalysis.
Sorin OLTEAN – Senior Technician (TS)
Dr. Marcela ROȘU – Established Researcher (R3)
Expertise: Nanocomposites, Photocatalytic processes, Environmental Chemistry.
Dr. Raktim Abha SAIKIA – First Stage Researcher (R1) Post Doc
Expertise: Organic Synthesis, Synthetic Methodology, Arylation Method, Multi-step Reactions, NMR spectroscopy.
Dr. Crina SOCACI – Leading Researcher (R4)
Expertise: Organic Chemistry, Physical Chemistry, Materials Science.
Dr. Alexandru TURZA – First Stage Researcher (R1)
Expertise: X-ray diffraction, Small angles X-ray scattering.
Alexandra URDA – Scientific Research Assistant (ACS)
Expertise: Nanostructured materials, Photocatalytic applications.
Dr. Adriana VULCU – Recognised Researcher (R2)
Expertise: Electrochemistry, Science of materials and nanomaterials.
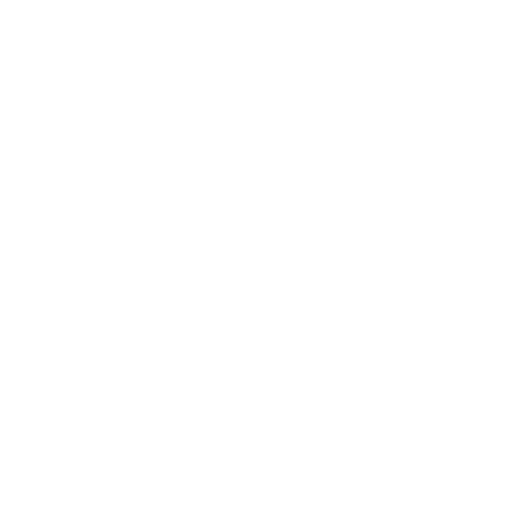
Carbon dioxide methanation
Maria Miheț, Monica Dan, Oana Grad, Gabriela Blăniță, Mihaela D. Lazăr
The efficient transformation of carbon dioxide in value added products is one of the most important and provocative area in now days catalytic research. Our group researches in this direction were focused on developing catalytic materials as Ni-Me/Al2O3 [1], Ni/MIL-101, Ni/UiO-66 [2] and Pt/UiO-66 [3] to be used in CO2 transformation in methane. All catalysts proved to be very active for CO2 activation, very selective for methane formation and also very stable and with high lifetime. All materials were prepared in our group and thoroughly characterized, both structural and functional. The catalytic tests show that the addition of small quantities of noble metals to classic Ni/Al2O3 catalyst, improve its proprieties for CO2 methanation [1]. Using MOF as catalyst support a much better Ni and Pt dispersion and stabilization on catalyst surface were obtained, with beneficial effect on catalyst activity and stability [2,3].
[1] Maria Miheț, Mihaela D Lazăr, Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion, Catal. Today, 306 (2018) 294-299
[2] Maria Miheț, Oana Grad, Gabriela Blăniță, Teodora Radu, Mihaela D. Lazăr, Effective encapsulation of Ni nanoparticles in metal-organic frameworks and their application for CO2 methanation, Int.J.Hydrogen Energy, 44 (2019) 13383-13396
[3] Maria Miheț, Gabriela Blăniță, Monica Dan, Lucian Barbu-Tudoran, Mihaela D. Lazăr, Pt/UiO-66 nanocomposites as catalysts for CO2 methanation processes, J. Nanosci. Nanotechnol., 19 (2019) 3187-3196
Electrochemical oxidation of methanol
Camelia Groşan, Adriana Vulcu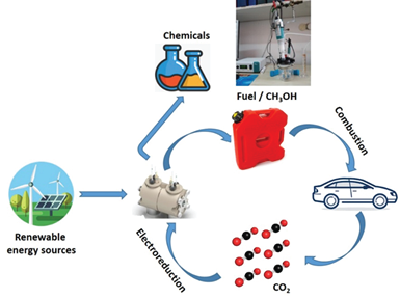
Platinum based catalysts are highly used as anode materials, but the scarcity of noble metal and its vulnerability toward carbon monoxide poisoning are major disadvantages for their commercialization. However, the platinum based catalysts activity can be enhanced by dispersion of platinum nanoparticles onto the carbon materials supports. Our research is focused on the development of an efficient catalyst for the methanol oxidation reaction (MOR) in alkaline media; thus, several electrodes modified with nanocomposites based on graphene and platinum nanoparticles have been tested for MOR. The best catalyst is a hybrid graphene-platinum (GrPt) material possesing the following characteristics: i) low platinum content (5.3 wt.%) – in the form of dispersed platinum nanoparticles and ii) platinum nanoparticles are coated with at least four layers of graphene. The material have been prepared by the catalytic vapour deposition method (CVD), while the composite, as GrPt ink, have been deposited on a glassy carbon electrode and its electrocatalytic activity in a methanol oxidation reaction was evaluated in a 1 M CH3OH/1 M NaOH solution. The results showed remarkable performance and durability of the GrPt during MOR in alkaline media (34.7% of the catalytic activity found after 500 cycles), suggesting that the growth of graphene on platinum nanoparticles is an efficient method to make a stable catalyst [1].
[1] Camelia Groşan, Teodora Radu, Alexandru R. Biriş, Monica Dan, Cezara Voica, Fumiya Watanabe, Alexandru S. Biriș, Adriana Vulcu, Platinum nanoparticles coated by graphene layers: a low-metal loading catalyst for methanol oxidation in alkaline media, J. Energ. Chem. 40 (2020) 81-88
Hydrogen absorption in 1 nm Pd clusters confined in MIL-101(Cr)
Gabriela Blăniţă, Dan Lupu
We report here the unprecedented modification of the hydrogen absorption/desorption properties of 1 nm Pd clusters relative to the bulk and nanoparticles down to 2–3 nm. These metal clusters have been synthesized by a facile double solvent impregnation method. They contain on average 33 atoms and are confined/stabilized into a metal-organic-framework with different metal loadings (5–20 wt%). This is the first time, to the best of our knowledge, that 1 nm Pd clusters are effectively confined into a MOF for high metal loadings. Such ultra-small nanoparticles are crystalline with the archetypical fcc structure of the bulk metal, as confirmed by both HR-TEM and in situ EXAFS. Hydrogen absorption/desorption properties of 1 nm Pd clusters have been characterized by both laboratory and synchrotron facilities. Under ambient conditions, 1 nm Pd clusters absorb hydrogen forming solid solutions instead of a hydride phase, as usually encountered for the bulk and Pd nanoparticles down to 2–3 nm. This can be understood by a decrease of the critical temperature of the two-phase region in the Pd–H phase diagram below room temperature. Moreover, the activation energy of hydrogen desorption from Pd clusters strongly decreases relative to bulk Pd. This suggests a change in the rate limiting step from surface recombination or b / a phase transformation usually encountered in bulk Pd to hydrogen diffusion into a and b phases in 1 nm clusters.
Malouche, G. Blăniţă, D. Lupu, J. Bourgon, J. Nelayah and C. Zlotea, Hydrogen absorption in 1 nm Pd clusters confined in MIL-101(Cr), J. Mater. Chem. A, 2017, 5, 23043-23052 (https://doi.org/10.1039/C7TA07159K)
Synthesis of graphene / graphene decorated with metallic nanoparticles by electrochemical exfoliation of graphite rods (prototype)
Valentin Mirel, Stela Pruneanu, Florina Pogăcean, Maria Coroș
The invention relates to a continuous, automatic system for controlling the process of electrochemical exfoliation of graphite bars by pulses of current. The system allows higher current densities to be used during exfoliation, without heating the electrolyte solution. The advantage of the proposed system is the use of three independent electrochemical cells, each of which has its own working parameters, such as the duration of the current pulses or the exfoliation time. The proposed assembly allows the digital choice of a high stability frequency range, from 1 Hz to 150 kHz. The voltage can be adjusted between 3.3 and 30 V. The switching elements of the 3 cells allow currents up to 5 A on each cell. The ratio given by the conduction / pause period of the current through the three cells can be set between 0 and 100%, independently on each cell. All the experimental parameters are easy to view/ control through the digital display and the 8 keys.
Patent Application: Automatic system for controlling the electrochemical exfoliation of graphite bars in order to obtain graphene, OSIM No.: A/00904 -16.11.2018.
Fabrication of graphene-modified electrodes employed for the electrochemical detection of tumor biomarkers, food additives and organic pollutants
Stela Pruneanu, Florina Pogăcean, Maria Coroș, Lidia Măgerușan, Valentin Mirel, Codruța Varodi, Crina Socaci
The electrodes obtained by screen printing offer several advantages over the traditional electrodes made of glassy-carbon or gold, because they are small in size, there is a possibility for mass production and also the possibility of being attached to a portable device that allows in-field measurements (Figure 2). In addition, the graphite inks used for the printing of the working electrode can be modified by adding materials or molecules, which allows the use of this type of electrode in biomedical analysis. A screen-printed electrode contains on the same substrate the working electrode where the electrochemical reaction takes place, the counter-electrode that allows the electrical circuit to be closed and the reference electrode that provides the reference value of the potential. The electrochemical performances of screen-printed electrodes modified with graphene were tested for the detection of a tumor biomarker (8-hydroxy-2-deoxiganosine) in standard laboratory solutions (PBS pH 6) as well as in artificial urine solutions and saliva.
Very good results were also obtained with glassy-carbon electrodes modified with graphene and used for the electrochemical detection of food additives (E110- Sunset Yellow; E102-Tartrazine) and organic pollutants (Bisphenol-A; Hydroquinone). The performances of the graphene-modified electrodes were superior to the unmodified ones, in terms of sensitivity, linear range and limit of detection.
[1] Florina Pogăcean, Maria Coroș, Lidia Măgerușan, Valentin Mirel, Alexandru Turza, Gabriel Katona, Raluca-Ioana Stefan-van Staden, Stela Pruneanu: Exfoliation of graphite rods via pulses of current for graphene synthesis: Sensitive detection of 8-hydroxy-2′-deoxyguanosine, Talanta, 196 (2019) 182-190
[2] Codruța Varodi, Florina Pogăcean, Maria Coroș, Marcela-Corina Roșu, Raluca-Ioana Stefan-van Staden, Emese Gal, Lucian-Barbu Tudoran, Stela Pruneanu, Simona Mirel: Detection of 8-Hydroxy-2’-Deoxyguanosine Biomarker with a Screen-Printed Electrode Modified with Graphene, Sensors, 19 (2019) 4297, doi:10.3390/s19194297
[3] Florina Pogăcean, Maria Coroș, Valentin Mirel, Lidia Măgerușan, Lucian Barbu-Tudoran, Adriana Vulpoi, Raluca-Ioana Stefan-van Staden, Stela Pruneanu: Graphene-based materials produced by graphite electrochemical exfoliation in acidic solutions: Application to Sunset Yellow voltammetric detection, Microchemical Journal, 147 (2019) 112-120
[4] Lidia Măgerușan, Florina Pogăcean, Maria Coroș, Crina Socaci, Stela Pruneanu, Cristian Leostean, Ioan Ovidiu Pană, Green methodology for the preparation of chitosan/graphene nanomaterial through electrochemical exfoliation and its applicability in Sunset Yellow detection, Electrochimica Acta 283 (2018) 578-589
-
- Max Planck Institute for Intelligent Systems, Stuttgart, Germany
Investigation of hydrogen cryo-adsorption on metal-organic frameworks (MOFs) - Université Paris Est, Institut de Chimie et des Matériaux Paris-Est (UMR7182), CNRS, UPEC, Thiais, France
Investigation of hydrogen adsorption/desorption properties of MOF-base hybrid materials - Polytechnic University of Valencia, Department of Chemistry, Valencia, Spain
Collaboration for catalytic utilization of MNPs@MOF composites - University of Bologna, Department of Industrial Chemistry “Toso Montanari”, Bologna, Italy
Collaboration for in-situ functional characterization of catalytic materials using DRIFTS (Diffuse Reflectance Fourier Transform Infrared Spectroscopy) - IUCN Dubna, Russia
Investigation of nanostructured materials by Small Angle Neutron Scattering (SANS) method - Università degli Studi del Piemonte Orientale – Alessandria, Italia
The synthesis of graphene and their use as support in enzymatic reactions. PhD student Candida Lorusso from this University spent 3 months (May-July 2019) at INCDTIM during which she used graphene as a support for carboxylesterase adsorption, and also testing the enzymatic degradation of a pesticide (2.2 -diclorovinil-dimethyl-phosphate) - INCEMC-București (the group of Prof. Raluca Stefan-van Staden)
The use of electrodes modified with graphene at the detection of tumor biomarkers - Universitatea de Medicină și Farmacie Iuliu Hațieganu din Cluj-Napoca (the group of Prof. Cecilia Cristea)
The use of graphene in the development of electrochemical sensors on flexible substrate
- Max Planck Institute for Intelligent Systems, Stuttgart, Germany